Phase Equilibria in Polymer Systems
Many polymer products are multi-component mixtures of polymers or of polymers and other compounds such as solvents, monomers and modifiers like plasticizers, tackifiers etc. Understanding the phase behavior of these mixtures is essential to obtain the desired blend properties.
In most cases, mixturers of polymers are not miscible, that is, only very few pairs of polymers are known to be miscible and then only in a narrow temperature and concentration range. The main factors that determine the phase behavior are
Average molecular weight and distribution of molecular weights
Structure and chemical composition of the polymers and additives/modifiers
Polymer concentration or polymer volume fraction in the blend
Temperature and pressure
Over the last seventy five years, a large number of models have been developed to describe the phase behavior of polymer blends and of polymers in solvents. A comprehensive description of these models can be found in the book "Thermodynamics of Polymer Blends" by Yuri Lipatov and Anatoly Nesterov.1 The models can be divided into four broad classes
Lattice models based on the Flory-Huggins theory such as the compressible regular2 solution free energy model (CRS)
Equation of state (EOS) models for lattice fluids such as Flory-Orwell-Vrij and Prigogine-Sanchez-Lacombe model
Free-volume activity coefficient models, based on UNIQUAC/UNIFAC group contribution approach
Models based on perturbation theory, such as the perturbed-chain statistical associating fluid theory (PC-SAFT)
The thermodynamic driving force for mixing is the the osmotic pressure Π. This quantity is always positive. However, for large molecules, Π is relative small. This follows dircetly from the osmotic pressure relationship (c → ∞):3
Π ≈ RT c Mn-1
where c is the polymer concentration which is equal to the polymer volume fraction divided by its specific volume, c = φp / vp and Mn the number average molecular weight.
The osmotic pressure rapidly decreases with increasing molecular weight, Mn (molecule size), and is very small for high molecular weights. Since mixing of polymers is an endothermic process, at least for the majority of polymer blends, most pairs of polymers are not miscible; or in other words, blends of two or more polymers are only miscible if special exothermal interactions between the repeat units of the two diffferent polymers are present, like hydrogen bonding, or when the polymers have a similar chemical structure (solubility parameters).
From the thermodynamic models of polymer solutions and polymer blends the conditions (temperature, pressure, composition) under which phase separation occurs can be predicted. The phase diagrams for two components can resemble any of those shown below.
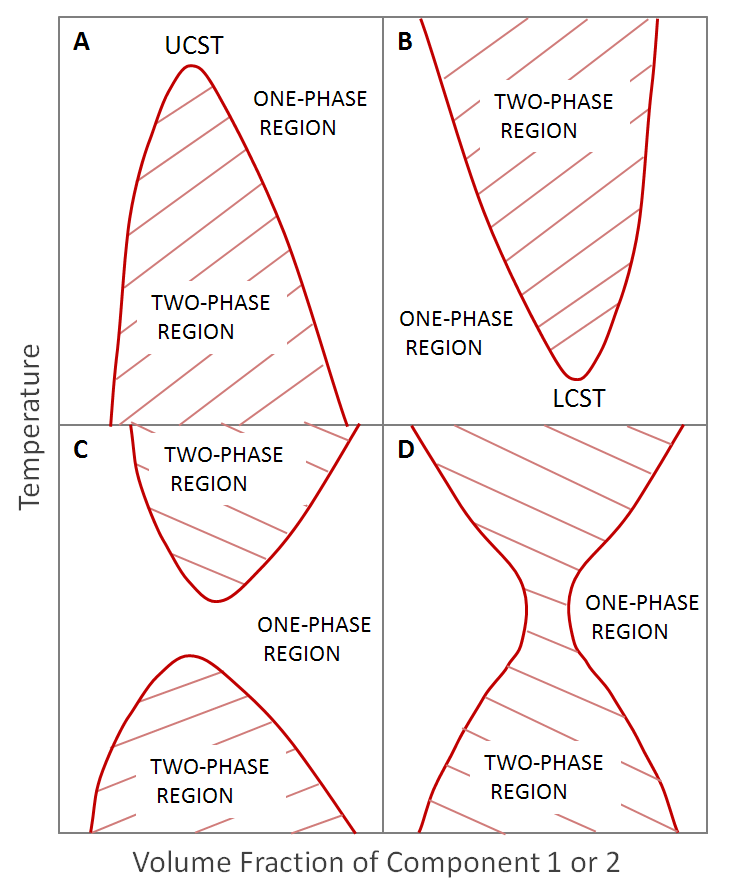
The clasical Flory-Huggins theory of mixing predicts only one region of immiscibility with an upper critical solution temperature. However, for some polymer blends, there are two regions of immiscibility; one is characterized by an upper critical solution temperature (UCST) and the other by a lower critical solution temperature (LCST). A third polymer blend system shows one continuous region of immiscibility extending from low to high temperatures with miscibilty at (very) low and high polymer volume fractions.
References & Notes
Y.S. Lipatov, A.E. Nesterov, Thermodynamics of Polymer Blends, Basel 1997
The word regular implies that all molecules (or repeat units of the polymer) mix in a completely random manner, that is, in a regular solution of composition φ1 and φ2 the probability that a neighbor of a given molecule is of type 1 is given by its volume fraction φ1 in the mixture.
Gert R. Strobl, The Physics of Polymers, 1st Edition Springer Berlin Heidelberg 2007